Actinic keratoses†‡
Natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial
Key personnel of the Department of Veterans Affairs Topical Tretinoin Chemoprevention (VATTC) Trial include the following: Study Chairman's Office: Martin A. Weinstock (Chair) and Kimberly Marcolivio (Providence, RI). Executive Committee: Martin Weinstock (Providence, RI), Stephen Bingham (Perry Point, Md), John DiGiovanna (Providence, RI), Russell Hall (Durham, NC), Mark Naylor (Oklahoma City, Okla), J. Richard Taylor (Miami, Fla), Julia Vertrees (Albuquerque, NM), and Clifton White (Portland, Ore). Clinical Centers: Durham, NC (Russell Hall and Deborah Hannah); Chicago, Ill (Hines) (David Eilers, Tehming Liang, Nadia Sakla, and Ann Kreuger); Long Beach, Calif (Gary Cole, Edward Jeffes, and Terri Labrador); Miami, Fla (J. Richard Taylor, Robert Kirsner, Jonette E. Kerri, Anna G. Falabela, and Margarita Givens); Oklahoma City, Okla (Mark Naylor, Mary Beth Benson, and Lisa Perry); and Phoenix, Ariz (James Kalivas, Catherine Yanni, Selma Targovnik, Janet Austin, and Susan Collier). Cooperative Studies Program Coordinating Center (Perry Point, Md): Joseph F. Collins, Stephen Bingham, Beverly Calvert, Philip Connor, Colleen Crigler, Dawn Davis, Pat Grubb, Judy Kelly, Gail Kirk, Karen Lawson, Linda Linzy, Lorrine Palmer, and Maxine Rhoads. Cooperative Studies Program Clinical Research Pharmacy Coordinating Center (Albuquerque, NM): Mike Sather, Erica Copeland, Carol Fye, William Gagne, Patricia Grimes de Naranjo, Chad Messick, and Julia Vertrees. Dermatopathologists: Michael Piepkorn (Bellevue, Wash) and Clifton White (Portland, Ore). Data and Safety Monitoring Board: Robert Lew (Boston, Mass), Irwin Braverman (New Haven, Conn), Bernard Cole (Lebanon, NH), Richard Kalish (Stony Brook, NY), David McLean (Vancouver, BC, Canada), and Bruce Thiers (Charleston, SC).
This article is US Government work and, as such, is in the public domain in the United States of America.
Abstract
BACKGROUND:
Actinic keratoses (AKs) are established as direct precursors of squamous cell carcinoma (SCC), but there is significant controversy regarding the rate at which AKs progress to SCC. The authors of this report studied a high-risk population to estimate the risk of progression of AK to SCC and to basal cell carcinoma (BCC) and the risk of spontaneous regression of untreated AKs.
METHODS:
Data were obtained from participants in the Department of Veterans Affairs Topical Tretinoin Chemoprevention Trial. Participants were examined every 6 months for up to 6 years. At each examination, the locations on the face and ears of clinically diagnosed AKs and lesions scheduled for biopsy were marked, and high-resolution digital photographs were taken. These photographs were used later to map and track the presence, absence, or biopsy of each AK across visits.
RESULTS:
In total, 7784 AKs were identified on the face and ears of 169 participants. The risk of progression of AK to primary SCC (invasive or in situ) was 0.60% at 1 year and 2.57% at 4 years. Approximately 65% of all primary SCCs and 36% of all primary BCCs diagnosed in the study cohort arose in lesions that previously were diagnosed clinically as AKs. The majority of AKs (55%) that were followed clinically were not present at the 1-year follow-up, and the majority (70%) were not present at the 5-year follow-up.
CONCLUSIONS:
In the current study, the authors quantified the malignant potential of clinically diagnosed AKs for both SCC and BCC, although many did not persist, and the results suggested that AKs may play a greater role in the overall burden of keratinocyte carcinomas than previously documented. Cancer 2009. Published 2009 by the American Cancer Society.
Actinic keratoses (AKs) are dysplastic keratinocytic lesions confined to the epidermis that are caused by ultraviolet (UV) radiation.1 They are 1 of the most common conditions treated by dermatologists,2 with an estimated prevalence of 39.5 million in the US in 2004 and annual costs totaling $1.04 billion.3 Although the most common reason for treatment is prevention of malignancy, lesions also are treated for cosmetic purposes and to provide relief from symptoms, such as tenderness or itch. It is generally accepted that these lesions can be direct precursors of squamous cell carcinoma (SCC), but there has been a paucity of investigations into the frequency of malignant transformation; thus, there is significant controversy over the rate at which AKs progress to SCC. Annual rates of transformation ranging from 0.025% to 20% have been reported,4 yet we are aware of only 1 study that directly quantifies this risk using primary data.5 That study was conducted in a general population sample, and the maximum follow-up of individual lesions was 1 year. The dearth of direct study of this phenomenon is remarkable.
For the current investigation, we used prospectively collected data from 1 center of a randomized, multicenter trial in a high-risk population with up to 6 years of follow-up, including photography and dermatologist examination, to estimate the risk of progression of AKs to keratinocyte carcinomas (KCs) and to assess the natural history of AKs.
MATERIALS AND METHODS
Data were collected from the participants at 1 of the 6 sites of the Department of Veterans Affairs (VA) Topical Tretinoin Chemoprevention (VATTC) trial, a randomized, multicenter trial of topical tretinoin 0.1% for the prevention of KCs of the face and ears. Additional details of that study are described elsewhere.6, 7 Although quantifying the times to onset of new basal cell carcinoma (BCC) or SCC were the primary focus of the trial, understanding the natural history of AKs was a secondary objective and, for this purpose, a subprotocol was implemented for the 182 participants at 1 site (the Oklahoma City VA Medical Center). All participants had been diagnosed with ≥2 KCs in the 5 years before enrollment in the study and hence represent a high-risk population. The study dermatologist (an experienced clinician who had been practicing clinical dermatology actively for 14 years) examined all participants at approximately 6-month intervals. There was no specific treatment of AKs (other than biopsy if they became bothersome or clinically suspect for KC) during the trial, although all participants were offered free sunscreen and encouraged to use it. Participants were queried at each visit regarding biopsies and dermatologic treatments that took place outside of the Oklahoma VA Medical Center. Participants did not report any biopsies and only reported 38 treatments outside of the VA (7.1% of all treatments) during the trial. During each examination, a standardized set of 3 high-resolution digital photographs of the face and ears was taken. Then, all AKs were identified by clinical criteria and were marked in red on the patient's face or ears. Lesions that were suspected carcinoma were scheduled for biopsy and marked in black. The photographs then were repeated to document these markings for later analysis. The investigators did not refer to images from previous visits during subsequent visits. After completion of the trial, the photographs were used to evaluate the presence, absence, or biopsy designation of each distinct face/ears lesion at each study visit. All lesions that were biopsied from the face or ears were evaluated by a local pathologist and by 1 of 2 central reference dermatopathologists who were blinded to the original diagnosis. The interobserver reliability of these diagnoses is documented elsewhere.8 The diagnosis made by the central reference dermatopathologists was used for study purposes. Only biopsies of lesions that once had been photographed and marked as AKs were included in our analyses of AK prognosis. AKs that were not located on the face or ears were excluded from all data considered in this study.
Statistical Analysis
Data were analyzed using Stata SE version 8 (StataCorp, College Station, Tex). The risk of progression was calculated using the Kaplan-Meier method. This study was approved by the relevant external review committees for research involving human participants.
RESULTS
Of 182 participants who were randomized at the Oklahoma City site of the VATTC Trial, 170 participants (93%) were photographed at a minimum of 2 study visits, and 1 individual was excluded because of ambiguity in the markings. The remaining 169 participants constituted the study cohort for this report. The mean age of the 169 participants was 68 years (median age, 70 years; range, 44-84 years), and 160 participants (95%) were men. Participants were followed for a mean of 42 months (median, 42 months range, 4-71 months) and were examined a mean of 7 times (median, 7 examinations; range, 2-16 examinations). At baseline, 160 participants (95%) had at least 1 AK. In total, 7784 distinct AKs were identified during the study, and 1960 of those AKs (25%) were diagnosed on the first visit. The number of AKs per participant that were diagnosed on the first visit ranged from 0 to 76 (mean, 12 AKs; median, 7 AKs) (Fig. 1). The total number of AKs observed per patient ranged from 1 to 162 (mean, 46 AKs per patient; median, 42 AKs per patient) (Fig. 2). Approximately 50% of the AKs were located on the temples and cheeks. The distribution of AKs by anatomic site is illustrated in Figure 3.
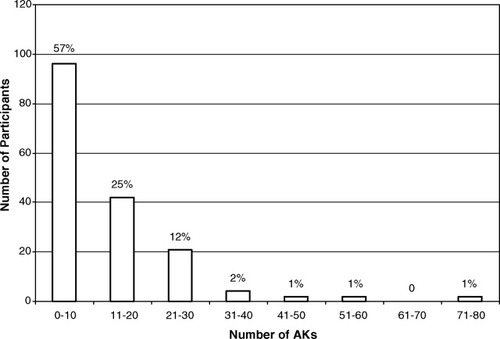
The number of actinic keratoses (AKs) on the face or ears per participant at the time of enrollment.

The total number of actinic keratoses (AKs) on the face or ears per participant.

Site distribution of total actinic keratoses on the face and ears.
During follow-up, 411 of these AKs were biopsied, at which time 122 (30%) were diagnosed as primary SCC (91 invasive and 31 in situ), 76 (18%) were diagnosed as primary BCC, 159 (39%) were diagnosed as AK, 16 (4%) were diagnosed as recurrent KC, 16 (4%) were diagnosed as seborrheic keratoses, and the remaining 22 either had other or nonspecific diagnoses or no pathology was observed in the biopsy specimen.
The risk of progression of AK to primary SCC (invasive or in situ) was 0.60% (95% confidence interval [95% CI], 0.44%-0.82%) at 1 year and 2.57% (95% CI, 2.12%-3.12%) at 4 years. For primary invasive SCC, the risk was 0.39% (95% CI, 0.26%-0.57%) at 1 year and 1.97% (95% CI, 1.58%-2.47%) at 4 years. The risks are listed in greater detail in Table 1. Figure 4 includes a Kaplan-Meier plot of the time to diagnosis of SCC for these clinically diagnosed AKs.
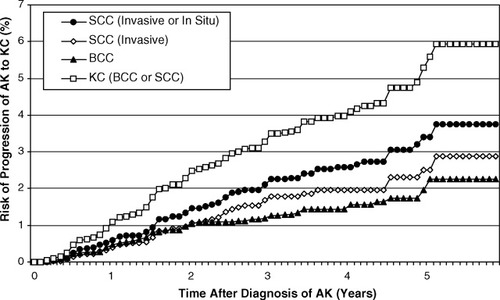
Results from a Kaplan-Meier estimate of the progression of actinic keratosis (AK) to primary keratinocyte carcinoma (KC). SCC indicates squamous cell carcinoma; BCC, basal cell carcinoma.
| Risk, %* | |||||
|---|---|---|---|---|---|
| Time After AK Diagnosis, Years | No. of AKs | Risk: All AKs, %* | 95% CI, % | Baseline AKs† | Nonbaseline AKs‡ |
| AK to SCC (invasive or in situ) | |||||
| 1 | 6015 | 0.60 | 0.44-0.82 | 0.89 | 0.48 |
| 2 | 4057 | 1.46 | 1.17-1.81 | 1.93 | 1.25 |
| 3 | 2688 | 2.25 | 1.85-2.74 | 3.13 | 1.84 |
| 4 | 1480 | 2.57 | 2.12-3.12 | 3.73 | 1.99 |
| 5 | 311 | 3.39 | 2.67-4.29 | 4.03 | 3.53 |
| AK to invasive SCC | |||||
| 1 | 6015 | 0.39 | 0.26-0.57 | 0.53 | 0.33 |
| 2 | 4057 | 1.06 | 0.82-1.37 | 1.31 | 0.95 |
| 3 | 2688 | 1.79 | 1.43-2.24 | 2.33 | 1.54 |
| 4 | 1480 | 1.97 | 1.58-2.47 | 2.57 | 1.69 |
| 5 | 311 | 2.50 | 1.90-3.30 | 2.88 | 2.50 |
| AK to BCC | |||||
| 1 | 6015 | 0.48 | 0.34-0.68 | 0.37 | 0.52 |
| 2 | 4057 | 1.04 | 0.80-1.34 | 0.94 | 1.08 |
| 3 | 2688 | 1.26 | 0.99-1.62 | 1.21 | 1.29 |
| 4 | 1480 | 1.56 | 1.21-2.02 | 1.58 | 1.55 |
| 5 | 311 | 2.27 | 1.54-3.33 | 2.64 | 1.55 |
| AK to KC | |||||
| 1 | 6015 | 1.08 | 0.85-1.36 | 1.26 | 1.00 |
| 2 | 4057 | 2.48 | 2.10-2.93 | 2.85 | 2.31 |
| 3 | 2688 | 3.49 | 2.99-4.06 | 4.31 | 3.11 |
| 4 | 1480 | 4.10 | 3.52-4.77 | 5.24 | 3.51 |
| 5 | 311 | 5.58 | 4.54-6.86 | 6.56 | 5.02 |
- AKs indicates actinic keratoses; 95% CI, 95% confidence interval; SCC, squamous cell carcinoma; BCC, basal cell carcinoma; KC, keratinocyte carcinoma.
- * Risk was calculated using the Kaplan-Meier method.
- † Baseline AKs were defined as AKs that were diagnosed at the initial examination at the time of enrollment.
- ‡ Nonbaseline AKs were defined as AKs that were not present at the enrollment examination but that developed during the course of the study.
A substantial number of the AKs were identified as BCC on follow-up. The risk of progression of AK to primary BCC was 0.48% (95% CI, 0.34%-0.68%) at 1 year and 1.56% (95% CI, 1.21%-2.02%) at 4 years. The risk of progression of AK to any KC (SCC or BCC) was 1.08% (95% CI, 0.85%-1.36%) at 1 year and 4.10% (95% CI, 3.52%-4.77%) at 4 years (Table 1) (Fig. 4). Eighty-one of 169 patients received topical tretinoin, but they did not differ in their rate of malignant transformation from that of patients who received the control cream at this center.
We used the log-rank test to compare the progression of AKs that were diagnosed at the initial examination (at enrollment; “baseline” AKs) with the progression of AKs that were diagnosed first at a follow-up examination. We observed that the baseline AKs had a significantly greater risk of progression to primary SCC (invasive or in situ; P = .02) but not to primary invasive SCC (P = .17), to primary BCC (P = .9), or to any primary KC (P = .06). Table 1 lists the risks of progression for baseline and nonbaseline AKs.
In total, 187 primary SCCs on the face or ears (139 invasive and 48 in situ) were diagnosed in this cohort after enrollment. Of these, 65% (65% of invasive SCCs and 65% of in situ SCCs) arose in previously clinically diagnosed and documented AKs. Of the 210 primary BCCs, 36% arose in previously documented AKs.
We estimated the proportion of baseline AKs (diagnosed during the first visit) that also were present at follow-up visits. For study purposes, we defined the 1-year follow-up visit for a given AK as the visit between 6 months and 17 months that was closest to 1 year. Similarly, the time intervals that defined the 2-year, 3-year, 4-year, and 5-year follow-up visits were 18 months to 29 months, 30 months to 41 months, 42 months to 53 months, and 54 months to 65 months, respectively. We observed that, among the AKs that were followed for the time included in the respective interval, 801 AKs (45%) were present at 1 year, 697 (42%) were present at 2 years, 409 (37%) were present at 3 years, 246 (31%) were present at 4 years, and 166 were present (30%) at 5 years (Table 2).
| Interval Since Baseline Examination, Years | Proportion Present | 95% CI | No. of AKs Present | Fraction Previously Absent* |
|---|---|---|---|---|
| 1 | 0.45 | 0.43-0.48 | 801 | 0.32 |
| 2 | 0.42 | 0.40-0.44 | 697 | 0.63 |
| 3 | 0.37 | 0.34-0.40 | 409 | 0.79 |
| 4 | 0.31 | 0.27-0.34 | 246 | 0.87 |
| 5 | 0.30 | 0.26-0.34 | 166 | 0.92 |
- 95% CI indicates 95% confidence interval; AKs, actinic keratoses.
- * The values in this column are the fraction of AKs present at baseline and at the time of the current evaluation (denominator) that were absent on at least 1 prior examination (numerator).
We noted that many AKs that were present at baseline and at a follow-up visit were absent on at least 1 visit between enrollment and that follow-up visit. Of the AKs that were present at 1 year, 257 (32%) were not identified on at least 1 visit; and, of the AKs that were present at 4 years, 246 (87%) were absent on at least 1 visit (Table 2).
DISCUSSION
AKs are 1 of the most common conditions treated by dermatologists.9 These superficial lesions generally are considered premalignant, and it is believed that they result from a clone of abnormal squamous cells caused by UV-induced gene alteration,10 although some investigators assert that they already contain the characteristics of malignancy and should be classified as such.11, 12 It is widely accepted that AKs may progress to invasive SCC and therefore may be treated to avoid possible morbidity and mortality. In the current study, we have quantified this potential for progression using prospective data from 1 center of a multicenter trial. Although we noted low risks of progression of AK to SCC, we observed that the majority of SCCs arose from AKs.
A wide range in the rates of malignant transformation exists in the literature. Quaedvlieg et al4 reviewed 875 studies of AKs and identified 62 randomized trials, observational studies, and reviews that mentioned the potential of AK progression to SCC. Of those 62 studies, 11 cited rates of transformation that ranged from 0.025% to 20% per year for individual AKs, but the genesis of these estimates sometimes is difficult to discern. The study by Marks et al5 used “grid maps” to mark the locations of AKs diagnosed on the head, neck, hands, and forearms so that the risk of transformation of individual AKs could be calculated. They noted that the annual risk of progression of an AK to SCC was <1 in 1000 (0.075% or 0.096%, depending on the classification of ambiguous lesions). Others used less satisfactory methods13 or could only estimate an upper bound of transformation risk14; and, in many reports, the source or justification of the quoted estimate was unclear or was not adequately justified.15, 16
We also mapped the individual AKs but used high-resolution digital photographs that were taken after all lesions were marked by a dermatologist to create an electronic digital record for each participant; in addition, we restricted our attention to the face and ears, where most KCs arise. Whereas the study by Marks et al5 included only 1 follow-up examination (at 1 year); in our study, we included 6-month follow-up intervals for up to 6 years, which allowed us to calculate a longer term risk of progression of individual AKs into SCC. We were able to examine the progression of AKs to BCC, which Marks et al did not report. We also were able to use the digital record to determine the presence or absence of each individual AK at every visit, which allowed us to assess the natural history of AKs.
A limitation of any study in which AKs are followed is the difficulty in consistently diagnosing an AK by clinical criteria. Weinstock et al17 observed substantial variation in AK counts among 7 experienced dermatologists who were investigators in the VATTC Trial. Other studies have observed between 81%18 and 94%19 histologic confirmation of randomly chosen, clinically diagnosed AKs; and, in a sample of patients with skin cancer, the confirmed proportion was only 74%.20 Of particular importance is that the frequency with which otherwise clinically normal, sun-damaged skin can be diagnosed as AK has not been well described.
Our study also was limited by the potential for ambiguity in determining correspondence between marked locations in serial photographs, especially if the participant had many AKs on the face or ears. It is particularly important that the AKs we followed were diagnosed clinically, so that some may have been KCs when they initially were photographed despite having the clinical appearance of AK. In addition, all participants were encouraged to use sunscreen, and it has been established that regular sunscreen use suppresses actinic neoplasia.21 Finally, because 95% of participants were men, the current results may not generalize to women.
Our estimate of progression of AKs to SCCs at 1 year was 6 to 8 times the estimate of Marks et al.5 The higher risk of progression most likely is because of our study population, which consisted of individuals at high risk (41% developed SCC in our study compared with 2% in the study by Marks et al). The difference observed between the risk of progression of prevalent AKs compared with incident AKs most likely is a function of the increased risk of progression with time, because the baseline AKs could have been present for any length of time before enrollment.
Although the risk of becoming an SCC is small for an individual AK, we observed that the majority (65%) of all SCCs in the study cohort arose in previously diagnosed AKs. Marks et al reported that a similar percentage (60%) of SCCs occurred at the site at which an AK had been recorded previously.5 Several other studies estimated the percentage of SCCs arising in AKs but did so by examining for the histologic presence of AKs in biopsy specimens of SCC as opposed to direct follow-up of AKs. Those studies indicated that between 72%22 and 82%23 of SCCs had concomitant AKs giving rise to or in close proximity to the SCCs.
We also observed that a substantial number of clinically diagnosed AKs were identified later as BCC at follow-up. Although it certainly is possible that these BCCs previously had been mistaken for AKs, it also is possible that these BCCs may have developed from AKs. Although this type of transformation has not been documented, there is documentation of KCs that have features of both BCC and SCC.24, 25 Clinically mistaking BCCs for AKs, and vice versa, is not uncommon and has been noted in the literature. One study26 indicated that 5% of 22 clinically diagnosed AKs were identified histologically as BCC. In another study, Kricker et al27 reported that 12% of 514 presumed BCCs were diagnosed histologically as AKs.
We observed that the majority of baseline AKs were not present clinically at the 1-year follow-up visit and by 5 years, only a small percentage still were present. Many of the AKs that were noted as present at the yearly follow-up visits were noted as absent at 1 point. Previous longitudinal studies also reported high turnover rates, with AKs that frequently developed, regressed, and recurred. In 1 longitudinal study, Frost et al28 reported that 346 (76%) prevalent AKs regressed when they were monitored over 12 months. In contrast Marks et al13 observed that only 485 of 1873 AKs (25.9%) regressed over 12 months. This disparity may have been caused by differences in the study populations or follow-up frequencies (both our study and the study by Frost et al used 6-month follow-up vs 12-month follow-up used in the study by Marks et al). Differences also may have been a result of the difficulty in diagnosing and following identified AKs over time.
AKs are a significant economic burden, and the annual costs of treatment-associated totaled $1.04 billion in 2004.3 Marks et al argued that, because few AKs progress to SCC, treating all AKs to prevent morbidity and mortality is difficult to justify by a cost-benefit analysis.5 We also observed a low risk of progression, but this risk was significantly greater than that estimated previously by Marks et al5 and increased steadily with time after initial diagnosis. It is important to note that this risk applies to individual AKs and, because most individuals with AKs have multiple lesions, the risk of a patient developing SCC from an AK can be much greater than the risk of transformation of an individual lesion.29 In addition to preventing morbidity and mortality, there are other important reasons for treating AKs, including symptom relief. A recent report indicated that the aggregate value for willingness to pay for symptom relief for all individuals with AKs was $2.4 billion.30
The results of the current study quantify the risk of progression of AKs on the face and ears to SCC in a high-risk population, document a significant rate of transformation to BCC, confirm a high rate of clinical regression, and indicate that approximately two-thirds of SCCs and one-third of BCCs in this context initially present as AKs. We suggest that the role of the clinically defined AK in the overall burden of KCs may be greater than previously appreciated.
Acknowledgements
We thank Kimberly Marcolivio, national project coordinator, and Lisa Perry, Oklahoma City data coordinator, for their outstanding work.
Conflict of Interest Disclosures
Supported by Cooperative Studies Program (CSP) Grant 402 from the US Department of Veterans Affairs Office of Research and Development.
Mr. Criscione was supported by a Summer Research Assistantship Grant from the Alpert Medical School of Brown University and by the Brown University Department of Dermatology.
Dr. Weinstock also was supported by Grants R01CA106592, R01CA106807, R25CA087972, and R01AR49342 from the National Institutes of Health.
The study sponsor was the CSP of the Office of Research and Development, Department of Veterans Affairs. In addition to providing funding to conduct the trial, the CSP provided the services of the CSP Coordinating Center (Perry Point, Md); the CSP Clinical Research Pharmacy Coordinating Center (Albuquerque, NM); and an outside review committee that initially reviewed the study for scientific merit, monitored study progress using various review committees, and gave final approval to this article.