Lymphedema beyond breast cancer†
A systematic review and meta-analysis of cancer-related secondary lymphedema
Presented in part at the International National Lymphedema Network Conference on Lymphedema, Nashville, Tennessee, November 1-5, 2006 and the American Society of Clinical Oncology Annual Meeting, Atlanta, Georgia, June 2-6, 2006.
Abstract
BACKGROUND:
Secondary lymphedema is a debilitating, chronic, progressive condition that commonly occurs after the treatment of breast cancer. The purpose of the current study was to perform a systematic review and meta-analysis of the oncology-related literature excluding breast cancer to derive estimates of lymphedema incidence and to identify potential risk factors among various malignancies.
METHODS:
The authors systematically reviewed 3 major medical indices (MEDLINE, Cochrane Library databases, and Scopus) to identify studies (1972-2008) that included a prospective assessment of lymphedema after cancer treatment. Studies were categorized according to malignancy, and data included treatment, complications, lymphedema measurement criteria, lymphedema incidence, and follow-up interval. A quality assessment of individual studies was performed using established criteria for systematic reviews. Bayesian meta-analytic techniques were applied to derive summary estimates when sufficient data were available.
RESULTS:
A total of 47 studies (7779 cancer survivors) met inclusion criteria: melanoma (n = 15), gynecologic malignancies (n = 22), genitourinary cancers (n = 8), head/neck cancers (n = 1), and sarcomas (n = 1). The overall incidence of lymphedema was 15.5% and varied by malignancy (P < .001): melanoma, 16% (upper extremity, 5%; lower extremity, 28%); gynecologic, 20%; genitourinary, 10%; head/neck, 4%; and sarcoma, 30%. Increased lymphedema risk was also noted for patients undergoing pelvic dissections (22%) and radiation therapy (31%). Objective measurement methods and longer follow-up were both associated with increased lymphedema incidence.
CONCLUSIONS:
Lymphedema is a common condition affecting cancer survivors with various malignancies. The incidence of lymphedema is related to the type and extent of treatment, anatomic location, heterogeneity of assessment methods, and length of follow-up. Cancer 2010. © 2010 American Cancer Society.
Lymphedema is a chronic, progressive, and often debilitating condition estimated to impact 2 to 3 million people in the United States,1 with secondary or acquired lymphedema accounting for the majority of cases. Secondary lymphedema is the result of obstruction or disruption of the lymphatic system, which can occur as a consequence of tumors, surgery, trauma, and radiation therapy, and the resulting mechanical insufficiency can lead to the accumulation of fluid in the interstitial tissues.
Lymphedema has been described as 1 of the most significant survivorship issues after the surgical treatment of breast cancer and in this population has been documented to have significant physical,2 functional, quality of life,3 and economic consequences.4 The reported incidence of lymphedema after breast cancer treatment varies widely, ranging from 6% to 63%,5, 6 depending on the population studied, measurement criteria used, and the reported length of follow-up.
Apart from breast cancer, secondary lymphedema has been reported as a consequence of treatment for several solid tumors, including melanoma, head and neck, gynecological, and genitourinary malignancies. A common feature among these solid tumors is the pattern of disease progression, with the development of regional lymphatic metastases often occurring before distant metastatic disease. Particularly in the absence of effective systemic therapies, the oncologic principles for treating regional lymph node metastases in patients with these tumors often include complete lymph node dissection of the involved lymph node basin, whether it be axillary, inguinofemoral, or pelvic (iliac/obturator). Although soft tissue or bone sarcomas rarely metastasize to regional lymph nodes, primary sarcomas of the extremities and the treatment of such tumors can result in the obstruction or disruption of the lymphatic system, resulting in secondary lymphedema.
Risk factors for lymphedema have been examined primarily in patients with breast cancer. In this population, extent of surgery, tumor burden, and adjunctive treatments (eg, radiation therapy) have been identified as primary risk factors for breast cancer-related lymphedema.7 The adoption of sentinel lymph node biopsy in the pathologic staging of breast cancer, along with other malignancies, has drastically reduced the number of patients undergoing elective lymph node dissection; by allowing surgeons to avoid lymph node dissections in patients with lymph nodes that do not harbor metastases, the risk of developing lymphedema in lymph node-negative patients has likely decreased. However, secondary lymphedema has been shown to occur after even simple operations, such as wide local excision or sentinel lymph node biopsy.8
Acknowledging the lack of consistent, comprehensive lymphedema incidence estimates outside of the field of breast cancer, the aim of this study is to review the available body of oncology literature assessing post-treatment lymphedema in patients with other types of malignancies to broadly examine the incidence of lymphedema in the wider population of cancer survivors. In addition, we sought to identify risk factors associated with the development of lymphedema, to inform healthcare providers and promote awareness of this widespread and debilitating condition in the oncology community.
MATERIALS AND METHODS
The systematic review of the literature was performed in 2 phases. The initial phase was carried out by a reference research librarian, who searched 3 major medical indices (PubMed-MEDLINE, Cochrane Library databases, and Scopus) for articles published between 1972 and 2008. Ten search strategies were used, and for each search, “lymphedema” and “cancer” were used as keywords, along with specific malignancies: colorectal; gynecological including cervical, uterine, vulvar; genitourinary including prostate, penile, and bladder; sarcoma; melanoma; and head/neck cancers. The initial searches resulted in 1099 entries, which were then imported into Endnote X2.0 (Build 3210, Thomson Corporation, Stamford, Conn) to remove duplicates (n = 230). In the second phase of the review, articles in languages other than English (n = 182) were excluded, as well as case reports, letters, and reviews (n = 314). A total of 373 eligible studies remained after the selection process (Fig. 1). After this, studies pertaining to lymphedema in patients with breast cancer (n = 36) or Kaposi sarcoma (n = 22) were excluded from further review.
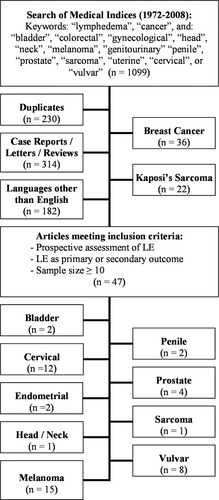
Search strategy and study selection are shown. LE indicates lymphedema.
A total of 315 articles were reviewed in detail with respect to predefined inclusion criteria requiring prospective assessment of lymphedema as a primary or secondary study outcome and a sample size of at least 10 patients. Because the primary focus of this review was to estimate the incidence of treatment-related lymphedema, retrospective reviews that documented prospectively collected lymphedema-related data were also included. To ensure the search was sufficiently comprehensive, articles from the archives of the authors were also examined, including reference lists from related articles. A total of 47 studies met inclusion criteria. The 47 studies were subsequently categorized according to type of malignancy, and extracted data included number of patients, extent of operation, postsurgical infections, use of radiation therapy, length of follow-up, criteria for defining lymphedema, measurement methods, and incidence of lymphedema.
Quality Control
A 14-item methodological checklist adapted from established quality criteria for systematic reviews was used to assess the quality of the 47 individual studies.9-11 Similar adaptations have recently been used in a systematic review of quality of life issues facing long-term breast cancer survivors.12 Each article was independently scored by 2 authors (R.L.A. and J.N.C.), with 1 point assigned for each criterion fulfilled. Score discrepancies were resolved through group consultation.
Statistical Analysis
The various clinical definitions, measurement methods, and follow-up reported when assessing lymphedema limited the methods available for summary and subgroup analysis. Weighted averages based on study size were calculated as pooled summary statistics for lymphedema incidence, and stratification methods were used to examine the individual effects of study characteristics on the summary estimates. When possible, subgroup analyses of studies reporting characteristics of patient treatment groups in sufficient detail were examined separately and recorded along with their respective reported incidence of lymphedema. Study characteristics designated a priori for examination included sample size, type of lymphedema assessment (ie, subjective vs objective), measurement methods (circumference vs water displacement), and length of follow-up. The effects of postsurgical infections, radiation therapy, and deep pelvic lymph node dissections were also examined when data were available.
Meta-Analysis
For the meta-analysis, summary incidence estimates were obtained using a Bayesian random effects model assuming a binomial distribution of the outcome.13 WinBUGS 1.4.214 was used to perform Markov chain Monte Carlo simulations. With the first 5000 draws discarded, only the next 45,000 draws were used to obtain posterior estimates based on 3 separate chains with overdispersed starting values. The Brooks, Gelman, and Rubin convergence statistics15 were used to assess the models, and only properly converged models were considered further.
RESULTS
A total of 47 eligible studies were identified that examined secondary lymphedema in patients with melanoma (n = 15), gynecologic cancer (n = 22), genitourinary cancers (n = 8), head/neck cancers (n = 1), and sarcomas (n = 1). The reported incidence of postoperative lymphedema in these studies varied from 0% to 73%, and there was considerable heterogeneity among studies with respect to lymphedema measurement methods, lymphedema classification, and follow-up intervals (Tables 1-4). For the combined total of 7779 cancer survivors included, 15% were identified with lymphedema; however, the incidence varied widely according to the type of malignancy (P < .001). The median patient follow-up varied greatly among the studies, ranging from 6 to 118 months. Globally, the reported incidence of lymphedema was higher for studies with longer follow-up (r = 0.38, P = .02) (Fig. 2).
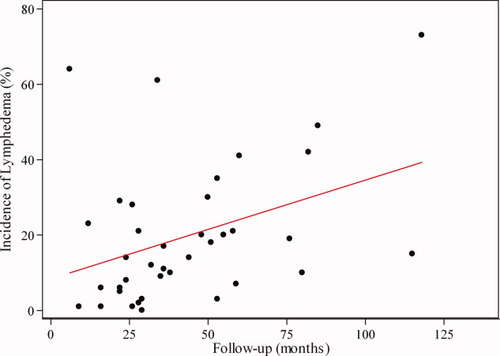
Scatterplot of studies by median follow-up (in months) and reported incidence of lymphedema with linear prediction is shown.
| Study | No. of Patients | Mean/Median Follow-up, mo | Lymphedema Measurement Method | LE Grade Criteria | Reported Incidence of LE, % | Quality Score |
|---|---|---|---|---|---|---|
| Axillary lymph node dissection | ||||||
| de Vries 200529 | 14 | 59 | Water displacement | >10% | 7 | 8 |
| Starritt 200433 | 107 | 36 | Water displacement/ circumference | >16% | 17 | 10 |
| Serpell 200319 | 33 | 22 | Subjective | — | 6 | 5 |
| Wrightson 200321 | 1688 | 16 | Subjective | — | 1 | 10 |
| Burmeister 200217 | 56 | — | Subjective | Grade 1: slight swelling; grade 2: obvious swelling; grade 3: treatment required; grade 4: incapacitating edema with ulceration | 39 | 10 |
| Lawton 200218 | 106 | 22 | Circumference | Mild: >2 cm; moderate: clinically apparent with reported heaviness; severe: obvious edema | 5 | 10 |
| Bowsher 198616 | 28 | 53 | Circumference | >2 cm | 3 | 4 |
| Urist 198320 | 98 | — | Circumference | Mild: 0-1.9 cm; moderate: 2-4 cm; severe: >4 cm; LE delineated at moderate | 1 | 5 |
| Inguinofemoral lymph node dissection | ||||||
| Brouns 200826 | 62 | 34 | Circumference | Discrete: 2-4 cm; moderate: 4-8 cm; severe: >8 cm | 61 | 7 |
| de Vries 200630 | 66 | 51 | Water displacement | Slight: 6.5%-20%; moderate: 20%-40%; severe: >40% | 18 | 10 |
| Wrightson 200321 | 784 | 16 | Subjective | — | 6 | 10 |
| Serpell 200319 | 27 | 22 | Subjective | — | 29 | 5 |
| Burmeister 200217 | 33 | — | Subjective | Grade 1: slight swelling; grade 2: obvious swelling; grade 3: treatment required; grade 4: incapacitating edema with ulceration | 66 | 10 |
| Lawton 200218 | 56 | 24 | Circumference | Mild: >2 cm; moderate: clinically apparent with reported heaviness; severe: obvious edema | 14 | 10 |
| Baas 199231 | 151 | 48 | Water displacement/ circumference | >6.5%; >3.5 cm | 20 | 5 |
| Bowsher 198616 | 44 | 53 | Circumference | >2 cm | 35 | 4 |
| Karakousis 198364 | 67 | 28 | Circumference | Slight: 1.5-2.5 cm; moderate: 3-4.5 cm | 21 | 9 |
| Urist 198320 | 58 | — | Circumference | Mild: 0-1.9 cm; moderate: 2-4 cm; severe: >4 cm; LE delineated at moderate | 26 | 5 |
| James 198232 | 33 | — | Water displacement/ circumference | >100 mL; >0.5 cm | 58 | 5 |
| Holmes 197727 | 84 | — | Circumference | >2 cm | 24 | 6 |
| Papachristou & Fortner 197765 | 81 | — | Circumference | >1 inch | 30 | 5 |
- LE indicates lymphedema.
- Axillary, n = 8 studies; inguinofemoral, n = 13 studies.
| Study | Type of Malignancy | No. of Patients | Mean/Median Follow-up, mo | Lymphedema Measurement Method | LE Criteria | Reported Incidence of LE, % | Quality Score |
|---|---|---|---|---|---|---|---|
| Jacobellis 200366 | Penile | 10 | 55 | Subjective | — | 20 | 4 |
| Henningsohn 200243 | Bladder | 224 | 115 | Subjective | — | 15 | 9 |
| Ravi 199328 | Penile | 234 | — | Circumference | Mild: 0-1.9 cm; moderate: 2-4 cm; severe: >4 cm; LE delineated at moderate | 21 | 5 |
| Kavoussi 199344 | Prostate | 372 | 9 | Subjective | — | 1 | 5 |
| Greskovich 199142 | Prostate | 65 | 29 | Subjective | — | 3 | 6 |
| Rainwater & Zincke 198850 | Prostate | 30 | 80 | Subjective | — | 10 | 4 |
| Lieskovsky 198046 | Prostate | 82 | — | Subjective | — | 18 | 5 |
| Clark 197839 | Bladder | 43 | — | Subjective | — | 23 | 3 |
- LE indicates lymphedema.
| Study | Type of Malignancy | No. of Patients | Mean/Median Follow-up, mo | Lymphedema Measurement Method | LE Criteria | Reported Incidence of LE, % | Quality Score |
|---|---|---|---|---|---|---|---|
| Carlson 200822 | Vulvar | 137 | 6 | Circumference | Mild: 0-2.9 cm; moderate: 3-5 cm; severe: >5 cm; LE delineated at moderate | 64 | 10 |
| Van der Zee 200867 | Vulvar | 383 | 35 | Subjective | — | 9 | 7 |
| Moore 200868 | Vulvar | 31 | 29 | Subjective | — | 0 | 9 |
| Tanaka 200752 | Cervical (42%), endometrial (47%), ovaries (11%) | 184 | 36 | Subjective | Stage I: tissue indents when pressed and holds indentation; stage II: tissue bounces back with no indentation; stage III: lymphostatic elephantiasis | 11 | 10 |
| Zhang 200769 | Vulvar | 57 | — | Subjective | — | 37 | 4 |
| Bellati 200570 | Vulvar | 14 | 58 | Common Toxicity Criteria | — | 21 | 7 |
| Judson 200471 | Vulvar | 61 | — | Subjective | — | 26 | 8 |
| Gaarenstroom 200372 | Vulvar | 101 | 26 | Subjective | — | 28 | 4 |
| Fujiwara 200341 | Cervical (66%), endometrial (34%) | 64 | — | Subjective | — | 11 | 8 |
| Bergmark 200236 | Cervical | 246 | 60 | Subjective | — | 41 | 7 |
| de Hullu 200173 | Vulvar | 106 | 118 | Subjective | — | 73 | 4 |
| Uno 200053 | Cervical | 98 | 76 | Subjective | — | 19 | 6 |
| Kridelka 199945 | Cervical | 25 | 32 | Subjective | — | 12 | 7 |
| Logmans 199947 | Cervical | 22 | 12 | Subjective/MRI | — | 23 | 5 |
| Snijders-Keilholz 199951 | Cervical | 220 | 38 | Subjective | RTOG/EORTC classification | 10 | 9 |
| Yeh 199954 | Cervical | 179 | 82 | Subjective | RTOG/EORTC classification | 42 | 4 |
| Chatani 199838 | Cervical | 128 | 85 | Subjective | Grade 1: mild symptoms; grade 2: treatment required; grade 3: surgery required | 49 | 7 |
| Werngren-Elgstrom 199423 | Cervical | 54 | — | Water displacement/ circumference | Slight: 5-9.9%/3.5-6 cm; moderate: 10-14.9%/6.5-12 cm; severe LE: ≥15%/≥12.5 cm | 41 | 7 |
| Orr 199149 | Endometrial | 168 | 26 | Subjective | — | 1 | 5 |
| Fiorica 199040 | Cervical | 50 | 28 | Subjective | — | 2 | 4 |
| Bilek 198237 | Cervical | 120 | 44 | Subjective | — | 14 | 6 |
| Martimbeau 197848 | Cervical | 402 | — | Subjective | Mild: cosmetic only; moderate: treatment required; severe LE: treatment does not help and occupational difficulties | 23 | 5 |
- LE indicates lymphedema; MRI, magnetic resonance imaging; RTOG, Radiation Therapy Oncology Group; EORTC, European Organization for Research and Treatment of Cancer.
| Study | Type of Malignancy | No. of Patients | Mean/Median Follow-up, mo | Lymphedema Measurement Method | LE Criteria | Reported Incidence of LE, % | Quality Score |
|---|---|---|---|---|---|---|---|
| Robinson 199125 | Sarcoma | 54 | 50 | Subjective | — | 30 | 7 |
| Wolff 200924 | Head/neck | 50 | 24 | Common Toxicity Criteria/late effects normal tissues criteria | — | 8 | 4 |
| Burmeister 200217 | Head/neck | 41 | — | Subjective | Grade 1: slight swelling; grade 2: obvious swelling; grade 3: treatment required; grade 4: incapacitating edema with ulceration | 5 | 10 |
| Urist 198320 | Head/neck | 48 | — | Circumference | Mild: 0-1.9 cm; moderate: 2-4 cm; severe: >4 cm; LE delineated at moderate | 0 | 5 |
- LE indicates lymphedema.
Type of Malignancy
A total of 15 studies were identified that assessed lymphedema in patients with melanoma between 1977 and 2008 (Table 1). In these studies, the reported incidence of lymphedema ranged from 1% to 66%, with an overall pooled incidence of 9% in 3676 patients. Melanoma patients were then stratified into 2 groups according to the anatomic region in which lymph node dissection was performed (ie, axillary and inguinofemoral). Six studies included both patients who had undergone axillary or inguinofemoral lymph node dissections,16-21; the dissection groups from these studies are listed separately in Table 1. The pooled incidence of lower extremity lymphedema after inguinofemoral lymph node dissection was higher (18%) than for upper extremity lymphedema after axillary lymph node dissection (3%). Twelve melanoma studies used objective measurements for lymphedema (ie, circumference or water displacement), with the remainder using subjective measures (n = 3). Follow-up for melanoma patients varied from 16 to 59 months, and the median quality score for these studies was 7 (range, 4-10).
A total of 8 studies examining lymphedema associated with genitourinary cancers, which included penile, prostate, and bladder cancers, were published from 1978 to 2008 (Table 2). Of the 1060 combined patients with genitourinary cancers, 11% were identified with lymphedema. Among those with penile cancer (n = 244), prostate cancer (n = 549), and bladder cancer (n = 267), the incidence of lymphedema was 21%, 4%, and 16%, respectively. Only 1 study in this group used objective measurement methods (circumference), whereas the remainder assessed lymphedema subjectively. The length of follow-up ranged from 9 to 115 months, and the median quality score for these studies was 5 (range, 3-9).
For the 22 articles examining lymphedema associated with gynecological malignancies published from 1978 to 2008 (Table 3), the incidence of lymphedema ranged from 0% to 73%. Of the 2850 patients with gynecological malignancies, 716 (25%) were identified with lymphedema (cervical, n = 1544, 27% with lymphedema; vulvar, n = 890, 30% with lymphedema; endometrial, n = 168, 1% with lymphedema). Nearly all of these studies used subjective measurement methods, with the only exceptions noted in 1 study that used water displacement and circumference measures to evaluate cervical cancer patients and in another that used circumference measures to assess patients with vulvar cancer.22, 23 The median quality score was 7 (range, 4-10) for studies of gynecological cancers, and follow-up ranged from 6 to 118 months.
Three studies were identified (1983 to 2008) that prospectively assessed lymphedema in patients with head and neck cancers (Table 4). Only Wolff et al reported mean follow-up (24 months),24 and the study by Urist et al was the only 1 to use objective assessment measures (circumference).20 The reported incidence of lymphedema ranged from 0% to 8%, and for the combined total of 139 patients, 4% were identified with lymphedema. The median quality score for these studies was 5 (range, 4-10). A single sarcoma study was published in 1991,25 where 54 patients were followed for 50 months using subjective lymphedema measurement criteria. The quality of the study was rated at 7, and the incidence of lymphedema was reported at 30%.
Treatment Factors
When available, patient-level data related to the surgical procedure performed (eg, inguinofemoral with or without pelvic lymph node dissection), postoperative complications, and treatment with radiation therapy were extracted and summarized. Twenty-two of the 47 articles identified a total of 2837 patients who had undergone pelvic lymph node dissection as a component of their cancer treatment. Overall, 22% of these patients developed lower extremity lymphedema. For those patients with melanoma undergoing iliac/obturator lymph node dissection (n = 132), the incidence of lymphedema was reported as 42%. For those undergoing pelvic lymph node dissection for genitourinary (n = 767) or gynecological cancers (n = 1938), the incidence of lymphedema was reported as 8% and 27%, respectively. A total of 18 articles examined the effect of radiation therapy on lymphedema, with a combined total of 1716 patients. Of those, 31% were identified with lymphedema. For those with melanoma undergoing radiation therapy (n = 106), 50% were noted to have lymphedema. For those with genitourinary (n = 284) or gynecological cancers (n = 1193) undergoing radiation treatment, the pooled incidence of lymphedema was reported as 16% and 34%, respectively.
Methods of Lymphedema Assessment
The most commonly reported objective measurement method was extremity circumference measurement (9 studies), with 7 studies using a difference threshold of >2 cm to define lymphedema.16, 18, 20, 22, 26-28 Two studies used water displacement methods exclusively for limb volume assessment, defining lymphedema thresholds of 6.5% and 10%,29, 30 and another 4 studies used both circumference and water displacement.23, 31-33 More subjective assessments of lymphedema included the Common Toxicity Criteria and the Late Effects Normal Tissues Scales,34, 35 but the majority used ad hoc clinical grading to define lymphedema such as skin-pinch tests or scales defined by the treating physician. Global comparisons revealed that patients were 91% more likely to be identified with lymphedema (relative risk, 1.91; 95% confidence interval [CI], 1.71-2.12; P < .001) when they were enrolled in studies using objective measurement methods (ie, volume and circumference measures) than those enrolled in studies using subjective (eg, self-report or provider observation) assessments.
Quality Assessment
The methodological quality of the 47 studies included in this review varied greatly. The maximum score possible from our quality assessment instrument was 14, but all ratings fell between 3 and 10 points, with a mean rating of 6.5 (standard deviation [SD], 2.2). The highest mean quality score was 7.3 (SD, 2.4) for melanoma studies, and the lowest mean quality score was 5.1 (SD, 1.8) for genitourinary cancers.
Meta-Analysis
The overall lymphedema incidence estimate derived from the random effect meta-analytic model (15.5%; 95% CI, 11.0-21.0) was nearly identical to the overall pooled incidence estimate of 15% (Table 5). However, greater differences in incidence estimates were observed in subgroup analysis. More specifically, for gynecologic malignancies and melanomas, differences between pooled and random effects incidence estimates ranged from 2% to 10%. However, all pooled incidence estimates fell within the 95% CIs of the random effects estimates.
| Group | No. of Studiesa | No. of Patients | Pooled Incidence, % | Range | Random Effects Incidence, % | 95% CI |
|---|---|---|---|---|---|---|
| Overall | 47 | 7779 | 15 | 0-73 | 15.5 | 11.0-21.0 |
| Upper extremityb | 8 | 2130 | 3 | 1-39 | 5.1 | 1.1-17.9 |
| Lower extremityc | 43 | 5456 | 20 | 1-66 | 19.9 | 14.3-26.8 |
| Head and neck | 3 | 139 | 4 | 0-8 | NA | — |
| Melanoma (overall) | 15 | 3676 | 9 | 1-66 | 16.3 | 8.6-27.8 |
| Upper | 8 | 2130 | 3 | 1-39 | 5.1 | 1.1-17.9 |
| Lower | 13 | 1546 | 18 | 6-66 | 28.0 | 17.2-42.2 |
| Genitourinary (overall) | 8 | 1060 | 11 | 1-23 | 10.1 | 3.2-25.2 |
| Bladder | 2 | 267 | 16 | 15-23 | 16.2 | 0-100 |
| Penile | 2 | 244 | 21 | 20-21 | 19.6 | 0-100 |
| Prostate | 4 | 549 | 4 | 1-18 | 4.8 | 0.1-71.7 |
| Gynecologic (overall) | 22 | 2850 | 25 | 0-73 | 19.6 | 11.1-31.0 |
| Cervical | 11 | 1544 | 27 | 2-49 | 21.8 | 11.6-35.8 |
| Endometrial | 1 | 168 | 1 | — | NA | — |
| Vulvar | 8 | 890 | 30 | 0-73 | 24.9 | 3.5-59.7 |
| Sarcoma | 1 | 54 | 30 | — | NA | — |
- CI indicates confidence interval; NA, not available.
- a Studies were included in the overall estimates that could not be discretely assigned to subcategories due to insufficient granularity of the data, and some individual studies were included in >1 category when sufficient data were available for multiple subgroups.
- b Includes melanomas only.
- c Includes genitourinary and gynecologic malignancies and lower extremity melanomas.
The meta-analytic techniques further revealed that lymphedema incidence estimates for melanoma were 16.3% overall, 5.1% for melanomas of the upper extremity, and 28.0% for melanomas of the lower extremity. For gynecologic malignancies, 19.6% incidence was observed for lymphedema overall, with 21.8% for cervical and 24.9% for vulvar malignancies. For sarcomas, head and neck tumors, and endometrial tumors, random-effect estimates could not be calculated because of insufficient sample size. Further model refinement to include potential covariates was also inhibited by missing data (median patient follow-up) or inadequate distribution of study characteristics (measurement method/criteria).
DISCUSSION
The overall incidence of lymphedema for cancer survivors in this review was 15.5%. Incidence rates varied widely among individual studies and according to primary tumor type. However, the observed disparity is more likely a function of the observed heterogeneity among study methods (eg, assessment methods and length of follow-up) than it is of the particular characteristics of the solid tumors reviewed. Increased lymphedema risk was observed for patients undergoing pelvic dissections (22%),18, 23, 26, 30, 36-54 as well as for those undergoing radiation therapy (31%).17, 24, 25, 33, 36-38, 40, 42, 43, 45, 46, 48, 50-54 The high incidence of lymphedema observed for gynecological malignancies (20%) is not surprising given that approximately 70% of the patients in these studies were reported to have undergone pelvic lymph node dissections and/or radiation therapy. However, an equal percentage of patients with genitourinary malignancies underwent pelvic dissections, and the overall incidence of lymphedema was only 10%. The differences may be related to the lower percentage of genitourinary cancer patients undergoing adjunctive radiation therapy (27%). Lymphedema was only reported in 4% of patients with head and neck malignancies and 5% for upper extremity melanoma, with far fewer patients undergoing pelvic dissections and radiation therapy in these groups. The higher incidence of lower extremity lymphedema after inguinofemoral lymph node dissection may be because of anatomic variation, with fewer associated lymphatic collateral pathways, or be related to a specific response to increased hydrostatic pressure in the disrupted lower extremity lymphatics.55
These findings are concordant with the primary risk factors for lymphedema identified in breast cancer survivors. Specifically, the surgical treatment for breast cancer, which often includes axillary lymph node dissection and/or mastectomy, is considered a major risk factor for lymphedema7, 56 The extent of lymph node dissection and tumor burden has also been shown to increase the risk of lymphedema.56 Over the past decade, less invasive surgical techniques such as sentinel lymph node biopsy and breast-conserving surgery have reduced the overall incidence of lymphedema; however, conservative estimates for incidence after sentinel lymph node biopsy are still in the range of 7%.57, 58 For high-risk patients treated with adjuvant radiation to a dissected axilla, the risk of subsequent lymphedema is greatly increased, with reported rates close to 45%.7 Other breast cancer-related lymphedema risk factors for which data were not available in this systematic review of nonbreast cancer lymphedema studies include patient age, obesity, tumor factors, lymph node status, the presence of postoperative seroma or infection, and the presence of venous obstruction. It is likely that these risk factors do not account for the entire at-risk population. Indeed, even outside the field of oncology, many of these are independent risk factors (eg, obesity, chronic venous insufficiency) for secondary lymphedema.59
Over the past decade there has been progress in the understanding of normal lymphatic function and the potential molecular pathways leading to lymphatic hypoplasia related to hereditary lymphedema. Several genes (FLT4, FOXC2, SOX18) have been shown to be associated and potentially responsible for the development of several variants of hereditary lymphedema.60 Mutations in vascular endothelial growth factor receptor-3 (VEGFR3) as well as the VEGF-C/VEGFR3 signaling systems have been identified as key components to both normal and pathologic lymphangiogenesis.61 It has been hypothesized that mutations or variations in the genes responsible for underlying genetically linked, anatomical, and physiological variations in the lymphatic system may also increase the risk of secondary lymphedema.62 The identification of such mutations could potentially have important therapeutic implications.
Not surprisingly, patients enrolled in studies using objective measurement methods were twice as likely to be identified with lymphedema compared with studies that used subjective scales. Currently, there is no gold standard measure or defining criteria for lymphedema assessment in the clinical setting, and the lack of a convenient, standardized, objective measurement method has resulted in reports of widely varying incidence. Although circumference measures may appear to be the most convenient, several problems exist, including problems with control of intra- and intermeasurer reliability. It is also time-consuming and requires considerable experience to obtain accurate measures. Although limb volume assessment using water displacement has historically been regarded as the most sensitive and accurate means to assess limb volume change, clinicians rarely use this cumbersome approach outside the research setting. To address these clinical challenges, several new devices have been introduced but have not yet become widely available. The perometer (JUZO, Cuyahoga Falls, Ohio) is an optoelectronic volumetry device that uses infrared light and an array of optoelectronic sensors to assess limb volume. In 2009, the US Food and Drug Administration approved the use of a bioelectrical impedance spectroscopy device (L-Dex U400, Impedimed, San Diego, Calif) for the clinical assessment of unilateral lymphedema of the arm in female breast cancer patients. Bioelectrical impedance spectroscopy measures tissue opposition (impedance) of body tissues to a low, alternating electric current over a range of frequencies to determine extracellular fluid volume.63 The magnitude of the impedance is used to determine the extracellular fluid volume and is expressed as an impedance ratio. Tissue tonometry is another method that measures the resistance of tissues to compression and quantifies tissue compliance. The degree of compressibility can then be correlated with limb swelling.
The strengths of this study include the large combined sample size for estimating lymphedema incidence, stratification by malignancy and extent of surgical resection, and the use of meta-analytic techniques. Specific eligibility criteria were used to exclude studies in which lymphedema was retrospectively assessed using medical records. Detailed patient-level data extraction was performed when possible to collate information related to radiation therapy and types of dissection. An assessment of methodological quality of the individual studies was included as an element of the systematic review to highlight the lack of methodological rigor in the current body of lymphedema literature. The weaknesses associated with this review can largely be attributed to the lack of consensus in the field of lymphedema. Because there are no precise consensus definitions or grading systems for lymphedema, clinicians and investigators have used a wide variety of independently developed, nonvalidated, subjective and objective diagnostic and rating criteria. In addition, limited patient-level data related to patient characteristics (ie, body mass index), radiation treatment, and postoperative complications did not allow for adjusted risk analyses. Furthermore, although a positive correlation was observed between length of follow-up and reported incidence, it is important to note that conclusions are limited by inherent variations among and between studies, including heterogeneity with respect to sample size, study quality, and clinical profile. Others, however, have previously reported a similar trend in studies of patients with breast cancer.6
In summary, cancer-related lymphedema is a common post-treatment condition. All persons undergoing lymph node dissection for the treatment of solid tumors should be considered at lifetime risk for developing lymphedema. In breast cancer patients, lymphedema has been described as an often overlooked, underdiagnosed, and undertreated condition, and the same can likely be said for patients with other malignancies. Early referral to lymphedema therapists for early intervention has been shown to reduce the risk of chronic lymphedema and to improve outcomes. As such, postoperative surveillance evaluations should at a minimum include observation for signs (eg, swelling) and assessment of lymphedema-associated symptoms (eg, heaviness), because early detection and intervention hold the greatest promise of reducing the incidence of this widespread condition. Additional prospective longitudinal studies that incorporate validated instruments and objective measurements are needed to further define the magnitude of this condition.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.