Seizures in patients with cancer
Abstract
Seizures are common in patients with cancer and either result from brain lesions, paraneoplastic syndromes, and complications of cancer treatment or are provoked by systemic illness (metabolic derangements, infections). Evaluation should include a tailored history, neurologic examination, laboratory studies, neuroimaging, and electroencephalogram. In unprovoked seizures, antiepileptic drug (AED) treatment is required, and a nonenzyme-inducing AED is preferred. Treatment of the underlying cancer with surgery, chemotherapy, and radiation therapy also can help reduce seizures. Benzodiazepines are useful in the treatment of both provoked seizures and breakthrough epileptic seizures and as first-line treatment for status epilepticus. Counseling for safety is an important component in the care of a patient with cancer who has seizures. Good seizure management can be challenging but significantly improves the quality of life during all phases of care, including end-of-life care.
Introduction
Seizures are common in patients with cancer as a consequence of brain tumors (both primary and metastatic) and as a result of adverse effects of treatments (surgery, radiation, systemic therapies) or can be provoked by systemic infections in immunocompromised patients or in those who have metabolic derangements resulting from the effects of systemic cancer (eg, hypocalcemia, hyponatremia). Seizures have the potential for physical and psychological harm as well as reduced quality of life. Formally, seizures are defined as the transient manifestation of signs and/or symptoms caused by synchronous neuronal or abnormal excessive brain activity.1 The most recent seizure classification groups seizures based on the type of seizure onset as focal onset, generalized onset, or unknown onset.1 Focal seizures can be with or without impaired awareness, whereas generalized seizures involve bilateral networks at onset, impair awareness, and often are accompanied by generalized convulsive activity.1 Another useful classification relates to the etiology of seizures, classifying them as provoked or unprovoked. Provoked seizures occur in the setting of temporary brain pathology, such as the effect of toxins and medications (eg, EtOH or benzodiazepine withdrawal), infections, or as a result of a metabolic derangement (eg, hyponatremia, hypoglycemia). Unprovoked seizures are caused by structural brain lesions, such as infarcts, hemorrhages, and tumors, or are the result of a genetic predisposition to experience seizures (genetic epilepsies).
After stabilizing the patient (see Management, below), the evaluation of a patient with cancer who presents with a first seizure should include a detailed history of the event (including any abnormal sensations reported by the patient before the event; a note of their state of awareness and a description of motor activity if the event was witnessed; a note of loss of urinary or stool continence during the event; the duration of the event; and the presence of confusion and/or agitation after the event) and a careful review of current medications and recent treatments. The patient should also undergo a neurologic examination to detect possible postictal deficits or subtle evidence of ongoing focal motor seizure activity. Brain imaging should be obtained, ideally using magnetic resonance imaging (MRI) with gadolinium, but a computed tomography scan of the head with contrast can be obtained instead if MRI is not available or cannot be readily obtained (MRI with gadolinium has higher sensitivity than computed tomography to detect brain lesions). Laboratory evaluations should include a complete blood count with differential, a complete metabolic panel, and toxicology studies. When clinically indicated (eg, meningeal signs on examination, blurring of optic disk margins on fundoscopy suggesting increased intracranial pressure), a lumbar puncture with opening pressure and cerebrospinal fluid studies (including cytology and flow cytometry to evaluate for leptomeningeal disease) should be obtained, but only after imaging has been obtained to evaluate for significant mass effect and the risk of parenchymal herniation (imaging and cerebrospinal fluid studies should not delay the initiation of empiric antimicrobials if infection is suspected).
An electroencephalogram (EEG) is indicated when patients with cancer have unexplained neurologic symptoms or confusion to rule out nonconvulsive status epilepticus or subclinical seizures.2 In patients who have returned to their neurologic baseline but report a history of intermittent neurologic deficits (eg, impaired awareness, difficulty speaking, intermittent paresthesias or weakness), long-term EEG monitoring might help identify an electrographic correlate for these spells. We recommend that, when an EEG is obtained, a neurology consultation is obtained as well to help evaluate the patient, review the EEG findings, and guide decision making regarding antiepileptic therapy. The presence or absence of brain lesions on imaging acts as a branching point for the management of patients with cancer who present with a first seizure.
Seizures in Patients Who Have Cancer Without Brain Lesions
When brain imaging does not reveal the presence of brain lesions or other acute intracranial pathology, such as hemorrhage or acute infarcts that could represent a seizure nidus, a review of recent treatments and an evaluation for systemic illness are warranted. Several systemic therapies are associated with seizures. Some of the most frequent culprits are listed with their common indications in oncology and recommendations regarding prophylaxis in Table 1.
| Drug | Mechanism of Action | Indications | Prophylaxis |
|---|---|---|---|
| Busulfan | Alkylating agent | CML, preconditioning for SCT | Premedicate with an AED |
| L-Asparaginase | Antimetabolite | ALL, AML | Not routinely used |
| Cisplatin | Platinum-based agent | Ovarian cancer, testicular cancer, bladder cancer | Not used |
| 5-Fluoracil | Antimetabolite | Colorectal cancer, pancreatic cancer, breast cancer, gastric cancer | Not used |
| Ifosfamide | Alkylating agent | Testicular cancer, sarcomas | Not used |
| Etoposide | Inhibitor of DNA replication | Testicular cancer, SCLC, medulloblastoma, pineoblastoma, ependymoma | Not used |
| Methotrexate (intrathecal) | Antimetabolite | Lymphoma | Not used |
| Chimeric antigen receptor T cells | Cell-based immunotherapy | B-cell ALL, B-cell lymphoma | Many protocols indicate prophylactic treatment with an AED |
| Blinatumumab | Bispecific T-cell engager Ab, anti-CD3/CD19 Ab | Multiple myeloma | Not used |
- Abbreviations: Ab, antibody; AED, antiepileptic drug; ALL, acute lymphocytic leukemia; AML, acute myelogenous leukemia; CML, chronic myelogenous leukemia; SCLC, small-cell lung cancer; SCT, stem cell transplantation.
Of the therapies listed in Table 1, we highlight chimeric antigen receptor T-cell therapies because these are increasingly being used for the treatment of refractory acute lymphocytic leukemia and lymphoma; and their neurotoxicity, most commonly characterized clinically by aphasia and encephalopathy, is frequently accompanied by EEG abnormalities (generalized periodic discharges) and seizures.3-5
Infections and metabolic derangements are other important causes of seizures in patients with cancer. Treatment-related immunosuppression puts patients who have cancer at increased risk of central nervous system infection with less common pathogens (Cryptococcus, human herpes virus 6). Hyponatremia is a well established cause of seizures in patients with cancer, particularly in those who have tumors originating in or involving the lungs (small-cell lung cancer), pleura, thymus, and brain. Hyponatremia secondary to the syndrome of inappropriate antidiuretic hormone can also be encountered in patients treated with cisplatin, cyclophosphamide, ifosfamide, vinca alkaloids, and imatinib.6 Hypocalcemia (often associated with tumor lysis syndrome), hyperglycemia (often secondary to corticosteroid treatment or pancreatic insufficiency), and hypoglycemia (secondary to tumor production of insulin or insulin-growth factors) all can be associated with seizures and should be screened for and corrected if identified.6 Seizures may result from either ischemic or hemorrhagic strokes associated with the cancer or its treatment.7
Finally, certain paraneoplastic syndromes, particularly those associated with limbic encephalitis, also are associated with seizures (see Table 2).8 Seizures in these patients tend to be refractory, and the identification of the syndrome based on other clinical symptoms should prompt aggressive treatment of the underlying malignancy (if known) as well as systemic immunosuppression. It is not uncommon for patients who present with seizures in the setting of a paraneoplastic syndrome to not have a diagnosed malignancy. In this case, appropriate investigations should be pursued based on the type of cancer suspected and, if initially negative, should be repeated periodically every 3 to 6 months for up to 4 years.9
| Antibody Target | Median Age at Presentation, y | Most Common Associated Tumor(s) | Other Symptoms Besides Seizures |
|---|---|---|---|
| Hu (ANNA-1) | 63 | SCLC | Memory loss, confusion |
| Ma-2 (Ta) | 64 | Testicular germ cell tumors | Memory loss, confusion |
| CV-2/CRMP-5 | 63 | SCLC, thymoma | Memory loss, confusion |
| NMDA-R | 23 | Ovarian teratoma | Psychiatric symptoms, choreiform movements |
| AMPA-R | 60 | Thymoma, breast | Psychiatric symptoms, memory loss, confusion |
| GABA-B-R | 62 | SCLC | Memory loss, confusion |
| LGI-1 | 60 | Thyroid, renal cell, NSCLC | Memory loss, myoclonus |
- Abbreviations: NSCLC, non–small-cell lung cancer; SCLC, small-cell lung cancer.
Seizures in Patients Who Have Cancer With Brain Lesions
Seizures are the presenting symptom in approximately one-third of patients with brain tumors, including both primary and metastatic tumors. During the course of their illness, many patients who have cancer experience seizures, with the incidence varying according to the type of tumor (Table 3). The location of the tumor can often predict the semiology of the experienced seizure. The main factors that influence the incidence of seizures are tumor location, type, grade, and location.10
| Type of tumor | Percentage With Epilepsy |
|---|---|
| DNET | 100 |
| Ganglioglioma | 80-90 |
| Low grade glioma | 75-80 |
| Meningioma | 22-60 |
| Glioblastoma | 29-60 |
| Metastases | 10-40 |
| Leptomeningeal disease | 10-15 |
| Primary CNS lymphoma | 10-20 |
| Gliomatosis cerebri pattern | 30-40 |
- Abbreviations: CNS, central nervous system; DNET, dysembryoplastic neuroepithelial tumor.
Lesions involving the cerebral hemispheres, especially those located on the cortical surface or associated with hemorrhage or edema, are more likely to result in seizure activity. Tumors involving the cerebral cortex, particularly the motor cortex, are more likely to produce seizures than tumors in the deep gray matter, although the latter can also lead to seizures because of surrounding edema and mass effect.11 Seizures can be a significant source of quality-of-life impairment for patients with brain tumors.12-15
In addition to the brain lesions themselves, seizures can also be a byproduct of the treatment provided, including surgery, radiation, and systemic therapies. On the basis of retrospective analyses of neurosurgical records, the estimated incidence of seizures after craniotomy is between 15% and 20%.16 Seizures can occur even in patients receiving prophylactic antiepileptic drugs (AEDs) and approximately two-thirds of these seizures occur during the first month postcraniotomy.17 It is a common neurosurgical practice to administer prophylactic perioperative and postoperative antiepileptics for up 1 or 2 weeks, although high-quality evidence to support this practice is lacking.18-20 Cranial radiation can lead to radiation necrosis in from 3% to 25% of patients, often presenting with seizures. Radiation necrosis is more common between 6 and 18 months postradiation and is more likely to develop with higher, concentrated doses (radiosurgery) and re-radiation.21, 22
Parenchymal brain metastases are encountered in 10% to 30% of patients with systemic cancer,23 and, of these, 70% to 80% present with multiple metastases (especially melanoma and lung cancer). In men, the most common primary tumor is lung cancer (particularly non–small-cell lung cancer), whereas, in women, breast cancer (particularly triple negative24) is the main offender. Melanoma has a strong propensity to metastasize to the brain (50% of patients with advanced melanoma develop brain metastases), and melanoma metastases are thought to be more epileptogenic given their high frequency of hemorrhage. Seizures are the presenting symptom in 10% to 40% of patients with brain metastases.25, 26 Brain metastases are usually found at the gray matter-white matter junction and present with seizures at a lower rate than primary parenchymal brain tumors because the latter are usually more infiltrative and disrupt local neuronal circuitry. Unless they have experienced a seizure, patients with brain metastases are not thought to benefit from treatment with antiepileptic medication25, 27 (see Management, below).
Primary brain tumors present with seizures at a higher rate than metastases, and lower grade tumors (grade I or II) present with seizures at a higher rate than higher-grade tumors (grade III or IV).26, 28 The higher incidence of seizures at presentation in low-grade primary brain tumors was first observed clinically by Bailey and Cushing in 192629 and today is considered a favorable prognostic factor for a patient with a newly diagnosed primary brain tumor.30 Multiple mechanisms have been proposed to explain the higher incidence of seizures in low-grade primary brain tumors. For most primary brain tumors, the abnormal electrical activity that leads to seizures is thought to arise from the peritumoral cortex—the interface between the invading tumor and normal brain—rather than from the tumor core itself.31 Tumors with neuronal elements, such as ganglioglioma, provide an exception to this rule. In gangliogliomas and other pediatric brain tumors, the high frequency (on the order of 60%) of BRAF V600E mutations has been linked to epileptogenic activity through increased expression of RE1-silencing transcription factor (REST), a regulator of gene expression for ion channel genes associated with epilepsy.32
In low-grade, diffuse gliomas (diffuse astrocytoma and oligodendroglioma), the higher incidence of seizures is thought to be related to isocitrate dehydrogenase (IDH) gene mutations,33 which are present in approximately 80% of these tumors.34 Mutant IDH converts α-ketoglutarate into D-2-hydroxyglutarate (D2-HG), an oncometabolite that, in addition to playing a role in oncogenesis through the alteration of epigenetic states,35 has a chemical structure similar to that of glutamate and, when secreted extracellularly, is thought to produce a proepileptic state by activating neuronal NMDA receptors, creating excitatory postsynaptic potentials and increasing the likelihood of action potentials.14
In high-grade gliomas, glutamate also plays an important role in epileptogenicity. SLC7A11/xCT, the catalytic subunit of system  (SXC) and a membrane antiporter that couples the efflux of glutamate with the influx of cystine, is upregulated in >50% of high-grade gliomas.36, 37 The upregulation of xCT leads to increased glutamate efflux, promoting excitotoxicity, seizures, and neuronal death.36, 37 Newly described glutamatergic synapses between neurons and tumor cells are also thought to mediate signaling associated with tumor growth.38-40 In gliomas, neuronal activity promotes tumor growth and invasion through glutamate activation of AMPA receptors in neuron-glioma synapses, and this growth appears to be abated by the AMPA receptor antagonist perampanel.38, 39 Similar neuron-metastasis glutamatergic synapses have been described in patients with breast cancer.40 These synapses also appear to promote tumor proliferation, in this case, through NMDA receptor signaling.40
(SXC) and a membrane antiporter that couples the efflux of glutamate with the influx of cystine, is upregulated in >50% of high-grade gliomas.36, 37 The upregulation of xCT leads to increased glutamate efflux, promoting excitotoxicity, seizures, and neuronal death.36, 37 Newly described glutamatergic synapses between neurons and tumor cells are also thought to mediate signaling associated with tumor growth.38-40 In gliomas, neuronal activity promotes tumor growth and invasion through glutamate activation of AMPA receptors in neuron-glioma synapses, and this growth appears to be abated by the AMPA receptor antagonist perampanel.38, 39 Similar neuron-metastasis glutamatergic synapses have been described in patients with breast cancer.40 These synapses also appear to promote tumor proliferation, in this case, through NMDA receptor signaling.40
It is worth mentioning that, despite our increased understanding of the electrical interplay between neurons and tumor cells and its implications for tumor proliferation, invasion, and survival,38, 40-42 to date, no clinical trials have demonstrated a survival benefit of antiepileptics as targeted tumor therapy in patients with brain tumors.43
Management
The mainstay of epilepsy management in brain tumor-related epilepsy (BTRE) is the use of AEDs. However, surgery, chemotherapy, and radiation therapy can both assist in the treatment of tumor-related epilepsy and treat the underlying cancer.44, 45
Chemotherapy
Chemotherapy with alkylating agents (including temozolomide and nitrosoureas) can lead to improved seizure control in patients with glioma, even when there is not a radiographic response on MRI. The favorable prognostic effect of seizure reduction is independent of age, neurologic symptoms, and previous chemotherapy.46, 47
Surgery
Epilepsy neurosurgery can be performed and differs from tumor neurosurgery because the goal is focused on seizure control. Epilepsy surgery consists not only in the maximal safe resection of the tumor but also the epileptogenic region surrounding the tumor. For otherwise benign tumors, this may be the only purpose of the surgery. Gross total resection with clear margins improves both tumor and epilepsy outcomes. Surgery can be effective in improving seizure control.48-50 Intraoperative electrocorticography may be used to guide complete resection of the epileptogenic area.51
Radiation Therapy
Radiation therapy can improve tumor-related epilepsy, but initially there may be an increase in the frequency of epileptic seizures or the appearance of de novo seizures. During acute treatment, there is increased edema and necrosis, which can lead to the worsening of seizures. This worsening often responds to corticosteroids (dexamethasone). Increased AED treatment may also be required. Radiation therapy can improve seizure control in the setting of low-grade glioma, even when there is an absence of tumor response on MRI.46 Of note, there is an increased risk of rash, including Stevens-Johnson syndrome, in patients treated concurrently with radiotherapy and either phenytoin, carbamazepine, or phenobarbital.52, 53
Antiepileptic Drugs
A single, unprovoked seizure in a patient with a brain tumor usually requires the indefinite use of an AED. But, in patients without a history of seizures, prophylactic AED use is not recommended; prophylaxis leads to more adverse events and does not decrease the risk of seizure.25, 54-56 However, in a high-risk situation, such as a patient with multiple hemorrhagic cortical metastases, there may be a benefit from prophylaxis with newer AEDs that lead to fewer adverse events than those previously studied.57
In a patient with a brain tumor, only 1 unprovoked seizure is required for the diagnosis of epilepsy. BTRE is a type of focal epilepsy. Focal-onset seizures may lead to impaired awareness and/or may progress to bilateral tonic-clonic activity. These seizure types can respond to treatment with any of the agents listed in Table 4 and with less commonly used AEDs. Principles used to select an AED are: efficacy, tolerability, safety, drug interactions, comorbidities, and cost. Potential interactions between the AED and any chemotherapeutic agent should also be considered when choosing an AED and monitoring levels. Even once a patient is seizure-free for 2 years, continued use of an AED is often required given the persistence of a seizure nidus—brain lesion or resection cavity—and the high risk of recurrent seizures, which can result in injury or worsen quality of life (for example, if the patient drives).58
| AED | Potential for Drug Interaction | Advantages | Disadvantages | Serious Adverse Events |
|---|---|---|---|---|
| Briveracetam | Low | Well tolerated, rapid titration, intravenous formulation | May be expensive, dizziness, psychiatric side effects | |
| Carbamazepine | High, CYP inducer | Mood stabilizer, inexpensive, intravenous formulation | Hyponatremia | Rash, bone marrow suppression, hepatotoxicity, increased risk of SJS with cranial radiationb |
| Clobazam | Lowc | Benzodiazepine with less sedation and less tachyphylaxis | Sedation, >80% protein bound; behavioral side effects; sialorhea | |
| Eslicarbazepine | Moderate, CYP inducer | Once-daily dosing | Hyponatremia | —b |
| Gabapentin | None | Well tolerated, treats neuropathic pain, low risk of rash, rapid titration | Weight gain, peripheral edema, less efficacious | |
| Levetiracetam | None | Well tolerated, low risk of rash, rapid titration, intravenous formulation, once-daily formulation | Avoid in patients at risk for depression, irritability, or aggression | |
| Lacosamide | Low | Well tolerated, rapid titration, intravenous formulation | Avoid if risk of heart block, dose-related dizziness, syncope | |
| Lamotrigine | Low | Well tolerated, mood-stabilizing and antidepressant properties once-daily formulation | Slow titration, insomnia, night sweats, arthritis | Rash, hypersensitivity syndrome, hemophagocytic lymphohistiocytosisb |
| Oxcarbazepine | Moderate, CYP inducer | Once-daily formulation | Hyponatremia | Rashb |
| Perampanel | Low | Dosed once daily, different mechanism of action (blocks glutamate activity at AMPA receptor) | May be expensive, 95% protein bound; hostility/aggression; weight gain | Rarely associated with homicidal ideation |
| Phenobarbital | High, CYP inducer | Oldest AED in use, commonly available, inexpensive, long half-life, intravenous formulation | Increased risk of bone loss; sedation, depression, hematologic toxicity | Increased risk of SJS with cranial radiation, hepatotoxicity, lupus-like reactions, aplastic anemia, increased risk osteoporosis |
| Phenytoin | High, CYP inducer | Inexpensive, intravenous formulation | Rash, gingival hyperplasia, nonlinear pharmacokinetics, glucose control challenging with diabetes mellitus | Increased risk of SJS with cranial radiation, hepatotoxicity, lupus-like reactions, aplastic anemia, increased risk of osteoporosisb |
| Pregabalin | None | Well tolerated, treats neuropathic pain, low risk of rash | Less efficacious; weight gain, peripheral edema | |
| Topiramate | Moderate | Reduces migraines, once-daily formulation | Anorexia, metabolic acidosis, language dysfunction, paresthesias | Nephrolithiasis, glaucoma, oligohydrosis |
| Valproic acid | High, CYP inhibitor | Reduces migraines, mood stabilizer; low risk of rash | >80% protein bound; weight gain, nausea, hair loss, tremor | Thrombocytopenia, hepatic injury, pancreatitis |
| Zonisamide | Low | Once-daily dosing | Anorexia, photosensitivity | Nephrolithiasis, glaucoma, oligohydrosis, rash |
- Abbreviations: AED, antiepileptic drug; CYP, cytochrome P450 enzyme; SJS, Stevens-Johnson syndrome.
- a Although this is not a comprehensive list, it represents the most common and serious adverse events. Depression is possible with all AEDs.
- b There is a risk of severe dermatologic reactions in patients of Asian descent who have the human leukocyte antigen-B*1502 allele.
- c Low indicates that the drug is not metabolized by the CYP system (SJS).
Although there are several AEDs from which to select, the use of AEDs in patients with cancer is complicated by potentially serious side effects and interference of AEDs with other commonly prescribed drugs, such as chemotherapeutic agents and corticosteroids. In selecting the appropriate AED, there are no randomized trials that have established the superiority of 1 agent over others in this situation. Despite preclinical trials, there is no consistent evidence to date that a particular AED is associated with improved outcomes.43
The older, first-generation AEDs have more drug interactions and side effects and are generally to be avoided. Phenytoin, carbamazepine, and phenobarbital induce cytochrome p450 and are referred to as enzyme-inducing AEDs. Enzyme-inducing AEDs should be avoided, especially when chemotherapy or radiotherapy is planned, because of the risk of decreased efficacy of chemotherapy and the increased risk of rash with radiotherapy. Enzyme-inducing AEDs also interact with corticosteroids and result in lower corticosteroid levels. Valproic acid is an older AED, but it inhibits, rather than induces, cytochrome p450. It may increase the levels of certain chemotherapeutic agents and can lead to hepatotoxicity or thrombocytopenia. Older AEDs, including valproic acid, are more heavily protein bound than the newer agents, leading to additional complications with treatment. These interactions have the potential to reduce the survival rate and increase the risk of relapse.59, 60
The newer (second-generation and third-generation) AEDs with no or minimal hepatic enzyme-inducing or enzyme-inhibiting properties are the preferred AEDs in cancer-related epilepsy (see Table 4). They have a more favorable safety profile with fewer problematic drug interactions. They are also better tolerated. However, even so, adverse drug events are common, occurring in from 30% to 40% of patients, and may be seen more frequently in those who have brain tumors.61, 62 Overall, studies have demonstrated that, on average, 23.8% of patients with brain tumors who are receiving AEDs experience adverse drug events that are severe enough to warrant a change in AED treatment.25 Common adverse drug events from AEDs in patients with brain tumors are changes in cognition, nausea or vomiting, behavioral changes, elevated liver enzymes, myelosuppression, ataxia, and rash.63 Side effects may reduce a patient's perception of their quality of life even more than seizure control.64 After starting or changing an AED, patients should be screened for adverse drug events during the titration period and at every subsequent visit.65
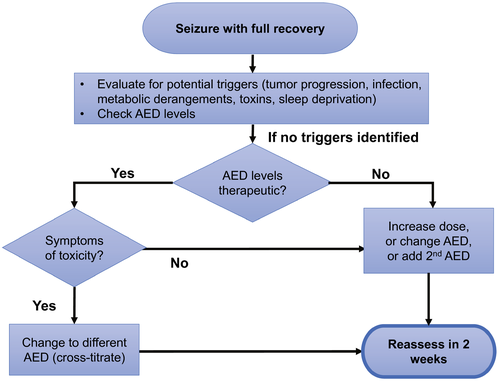
AED management begins with selection of the best agent for the patient, titrating up to the lowest effective dose. Approximately 50% of patients with tumor-related epilepsy will respond adequately to a single AED.66 The assessment and management of patients who experience another seizure while already taking an AED are illustrated in Figure 1. Nonadherence or AED changes are the most common causes of breakthrough seizures and status epilepticus in all patients who have epilepsy, including BTRE.67 Serum drug levels are helpful when initiating certain AEDs (eg, valproate, phenytoin) and when assessing nonadherence or drug interaction; otherwise, they have a limited role in the ongoing management of epilepsy. When it is unclear whether or not a patient's symptoms are related to AED toxicity, a serum level can be helpful because patients are more likely to have side effects with a high level. After a breakthrough seizure, a single AED should be optimized before changing to a different AED or adding a second agent.
Resistance to AEDs (ie, drug-resistant epilepsy) can be a major problem, and many patients will require the use of more than 1 AED. If combinations of 2 drugs fail, then triple AED therapy is used. However, at this point, it is unlikely that the patient will experience freedom from seizures using AEDs alone.68 The use of ≥3 AEDs is likely to increase toxicity and impair quality of life and is unlikely to result in better seizure control.
Levetiracetam is often favored as the first line AED for BTRE, given its effectiveness, good tolerability, and absence of drug interactions.69, 70 The exact mechanism of action is unknown, but it involves modulation of synaptic neurotransmitter release through binding to the synaptic vesicle protein SV2A in neurons. Tablet, liquid, extended-release, and intravenous formulations are available. No dosage change is required when changing formulations. However, adjustments in doses are needed in cases of renal failure. Levetiracetam can cause fatigue and neuropsychiatric side effects, including irritability (in 1%-10% of patients71), agitation, anxiety, and depression, even in patients without a pre-existing psychiatric history. Brivaracetam is 1 of the newest AEDs and is similar to levetiracetam, with a similar mechanism of action and both oral and intravenous formulations, but it is more expensive and has drug interactions.
Other commonly used AEDs for BTRE include other nonenzyme hepatic inducers, such as lacosamide and valproic acid. Lacosamide is a third-generation AED with a novel mechanism of action of selectively enhancing the slow inactivation of voltage-gated sodium channels. It has low protein binding, no induction or inhibition of hepatic enzymes, few drug interactions, and low incidence of side effects (however, because it can cause PR interval prolongation, it should not be initiated in patients with PR >200 msec). Lacosamide has tablet, liquid, and intravenous formulations and is generally well tolerated in patients with brain tumors.72, 73 Lamotrigine, perampanel, zonisamide, and clobazam are other good choices. Pregabalin and gabapentin may be less efficacious but can be helpful in add-on therapy, are well tolerated, and can be quickly up-titrated. Oxcarbazepine, Eslicarbazepine, and topiramate are efficacious but have a greater potential for drug interactions. With the use of new AEDs, cost is important to keep in mind.
Many patients with brain tumor inquire about the use of cannabis in the prevention of seizures. Cannabis has 2 main components: tetrahydrocannabinol (THC), which has psychoactive effects and can be used for anorexia and weight loss, and cannabidiol (CBD), which has no psychotropic effects, and there is a US Food and Drug Administration-approved CBD product for the treatment of seizures associated with 3 specific epilepsy syndromes (Lennox-Gastaut syndrome, Dravet syndrome, and tuberous sclerosis). To date, there is no evidence to support the use of CBD in adults without these syndromes. CBD is a powerful inhibitor of cytochrome p450 and may also induce other isoenzymes.74 CBD has as many adverse drug events as other conventional agents and is associated with side effects of fatigue, hepatitis, decreased appetite, diarrhea, and somnolence. Cannabinoids currently do not have an established role in tumor-related epilepsy, but studies are ongoing.75, 76
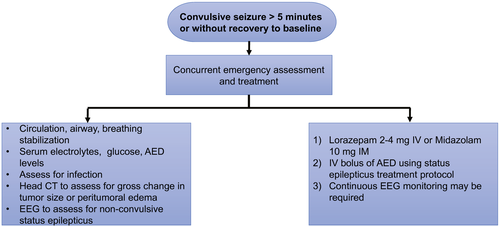
Patients who have brain tumors can develop status epilepticus, which is considered a neurologic emergency.77 The initial management is outlined in Figure 2. Status epilepticus can be either convulsive or nonconvulsive. Nonconvulsive status epilepticus can have nonspecific clinical manifestations, such as confusion, altered behavior, or a decreased level of alertness, which can be difficult to distinguish from other causes of altered mental status. Nonconvulsive status epilepticus can occur in patients with systemic cancer who do not have intracranial disease. Continuous EEG monitoring is required for guiding and optimizing treatment.
An important component in the palliative care of patients with cancer is the prevention and treatment of seizures. Many patients with brain tumors have seizures in the last month of life. Patients who have a history of seizures and high-grade gliomas are at particularly high risk, with a seizure recurrence risk as high as 76%, and 2.5% have status epilepticus.78 For these reasons, patients should continue to receive AEDs during hospice care. When swallowing is no longer possible, benzodiazepines can be provided through sublingual, buccal, intranasal, and rectal administration. Most AEDs can also be compounded into suppository formulations for rectal administration.79, 80
Other Treatments for Epilepsy
Non-AED treatments for epilepsy include dietary therapies, vagus nerve stimulation, deep-brain stimulation of the bilateral anterior thalami, and responsive neurostimulation. With the exception of vagus nerve stimulation, these therapies are not well studied in patients with brain tumors.81 The vagus nerve stimulator is an implanted medical device that stimulates the left vagus nerve in a closed-loop system to reduce seizures; it appears to be equally effective in patients with BTRE and those with other forms of epilepsy and is appropriate to consider in patients with BTRE who are not candidates for resection in whom imminent tumor progression is not expected.82 Dietary therapy for epilepsy consists of a high-fat, low-carbohydrate, ketogenic diet (KD) or a low-glycemic diet. A KD has long been recognized as safe and effective in decreasing seizures but can be difficult to maintain.83, 84 Preclinical studies have suggested positive effects of the KD on malignant glioma in animal models. However, there is a paucity of data on the efficacy of KD in improving survival and quality of life in patients with glioma.85
Counseling
Education and counseling regarding safety are vital in the care of patients with epilepsy. Patients who have brain tumors require counseling about the signs and symptoms of seizures. Patients with epilepsy have an increased risk of injuries and accidents, particularly drowning, burns, poisoning, adverse effects of medication, and traumatic brain injury.86 The risk of sudden unexpected death in epilepsy is approximately 1 in 1000 adults per year and 1 in 4500 children per year.62 The risk factors for sudden unexpected death in epilepsy include convulsive seizures and uncontrolled seizures. It is temporarily illegal to drive after a seizure. Discussions about driving are an important topic.87 The role of the physician in both reporting and determining the amount of time a patient has been seizure-free before driving varies from state to state, from physician discretion, to 3 months, to >1 year. In the United States, the regulations of each state can be found at epilepsy.com/driving-laws. Patients with epilepsy have a higher rate of depression, with approximately 30% of individuals who have BTRE developing clinically significant depression.88 There is also an increased risk of suicide among individuals with epilepsy, particularly at the time of diagnosis, and in individuals who have a history of depression. Treatment with selective serotonin and norepinephrine reuptake inhibitors is considered safe in individuals with epilepsy when used at therapeutic doses.89
Conclusions
Given the marked disability that can result from poorly controlled seizures, patients with cancer who have seizures, particularly those who have seizures in the setting of intracranial disease, benefit from being followed by a neuro-oncologist or a neurologist specializing in epilepsy. Good seizure management can be challenging but significantly improves the quality of life during all phases of care, including the end-of-life care, when appropriate treatment can prevent unnecessary hospital admissions and transitions of care.
Funding Support
L. Nicolas Gonzalez Castro was supported by the K12 Training Program in Nervous System Tumors (K12CA090354).
Conflict of Interest Disclosures
The authors made no disclosures.